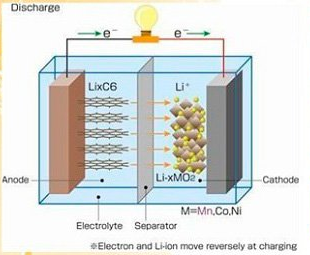
The influence of electrolyte on the performance of lithium battery
Electrolyte is a very important part of lithium-ion batteries. It can
affect many aspects of the performance of lithium batteries, mainly affecting
the following aspects:
1. Impact on battery capacity
The electrolyte affects the reversible capacity of the electrode material
to a large extent, because the charging and discharging process of the battery
is always the process of interacting with the electrolyte.
During the working process of lithium-ion batteries, there are a large
number of side reactions. These factors affect the reversible capacity to
varying degrees. Therefore, different types of raw materials, even different
models of different manufacturers, are required to configure electrolytes with
different formulations.
2. Impact on battery internal resistance and rate charge and discharge
performance
The sum of ohmic internal resistance, interface resistance, and
polarization internal resistance is the total impedance of lithium-ion
batteries. It is an important indicator to measure the performance of chemical
power sources and directly affects the battery's working voltage, working
current, output energy and power.
The ohmic resistance is mainly derived from the conductivity of the
electrolyte. The higher the internal resistance of the battery. Under normal
circumstances, the interface resistance is significantly higher than the ohmic
resistance.
Rate charge-discharge performance is an important indicator to measure the
capacity retention capacity of lithium-ion batteries under fast charge-discharge
conditions. The rate charge and discharge performance of the battery depends on
the internal resistance, which is closely related to the composition and
properties of the electrolyte.
3. Impact on battery operating temperature range
Among all environmental factors, temperature has the most obvious impact on
battery performance. Under low temperature conditions, the rate of electrode
reaction decreases, and the performance of the battery decreases significantly.
When the temperature is increased, the electrode reaction intensifies, but the
undesirable side reactions are also intensified at the same time, which affects
the performance of the battery. Therefore, the best temperature for battery
operation should be the temperature that is most conducive to electrode reaction
without obvious side reactions. The operating temperature range of lithium-ion
batteries is usually -10-45°C; the minimum operating temperature is generally
not lower than -20°C. The maximum operating temperature generally does not
exceed 60°C.
The main way to broaden the operating temperature range of lithium
batteries is to improve the conductivity of the electrolyte under low
temperature conditions and the stability under high temperature conditions.
4. Impact on battery storage and cycle life
The aging of lithium-ion batteries during long-term storage is the key to
battery storage performance. A commercial lithium-ion battery, even if it is
never used, has a storage life of only about 3 years. There are many reasons for
battery aging. The electrolyte affects or even determines the storage life of
the battery to a large extent.
5. Impact on battery safety
Lithium-ion batteries will deposit metal lithium on the surface of the
negative electrode under overcharge conditions, and a series of unsafe side
reactions will occur inside the battery, which brings significant safety
issues.
The source of the safety problems of lithium-ion batteries is still the
volatility and high flammability of the electrolyte itself. To fundamentally
eliminate the safety hazards of batteries, it is necessary to eliminate the
flammability of organic solvents, and develop safer or use electrolyte systems
that do not burn at all. This is a critical technical difficulty particularly
for large-scale, high-power-density lithium-ion batteries.
6. Impact on battery self-discharge performance
The appearance of impurities in the electrolyte is an important cause of
battery self-discharge. This is because the oxidation potential of impurities is
generally lower than the positive electrode potential of lithium-ion batteries,
which will continue to consume the active materials of the positive and negative
materials and cause self-discharge. Therefore, lithium-ion batteries have high
requirements on the composition and purity of the electrolyte.
7. Impact on battery overcharge and over-discharge behavior
Under some practical application conditions, when multiple lithium-ion
batteries are used in series to obtain a higher voltage, there is often an
obvious capacity mismatch. The battery pack will always have individual
batteries overcharged during charging and individual batteries during discharge.
The over-discharge of the battery will affect the life span and also bring
obvious safety hazards.
Electrolyte is an important way to prevent the battery from overcharging
and discharging. Most research is to establish an internal overcharge and
discharge protection mechanism inside the organic liquid electrolyte.

Lithium-ion battery (LIB) has become the main energy storage solution in
modern social life. Among them, lithium iron phosphate batteries are a perfect
substitute for lead-acid batteries, and are the first choice for grid-connected
peak shaving, off-grid energy storage, photovoltaic energy storage, UPS, data
center and other industries.