Lithium iron phosphate battery VS ternary lithium battery, SES Power
explains the differences between the two branches of lithium battery in
detail
In recent years, lithium-ion batteries have been widely used as the main
force of secondary rechargeable batteries. With the impact of global energy
demand, unstable petrochemical energy supply, and fluctuations in the
development of new energy, lithium-ion batteries have become increasingly
important.
Lithium iron phosphate and ternary lithium batteries are the two major
technical routes in lithium-ion batteries, and their battle has never stopped.
Lithium iron phosphate batteries, which can perfectly replace lead-acid
batteries, are the younger generation. In addition to capturing the market share
of lead-acid batteries, nickel-metal hydride batteries, and nickel-chromium
batteries, they are also accelerating the market for ternary lithium
batteries.
As SES Power with 20 years of rich experience in the customized lithium
battery industry, our products are mainly focused on energy storage system
integration and lithium batteries in special scenarios, such as fully
intelligent lead-acid replacement with Bluetooth or RS485 communication.
Products (lithium iron phosphate 12V100Ah, 12V200Ah), CCA2000A lithium iron
phosphate high-current starting lithium battery (direct replacement for
automotive lead-acid starting batteries), UPS high-voltage lithium battery
system (up to 860V), 3Kw~20Kw off-grid, grid-connected, island type Lithium
battery energy storage system, base station communication backup battery system
(standard 19 inches), full set of photovoltaic energy storage components, normal
charging and discharging lithium batteries under -40 degrees Celsius
environment, etc. We are very familiar with lithium iron phosphate batteries and
ternary lithium batteries. Let our senior engineers analyze the two major
branches of lithium batteries for you.
A: Lithium iron phosphate material and battery
The LiFePO4 with a three-dimensional spatial network olivine structure
forms a one-dimensional Li+ transport channel, which limits the diffusion of
Li+; at the same time, the octahedral FeO6 is connected at the top, making its
electronic conductivity low, and the polarization is relatively high during
high-rate discharge. big.
The charging and discharging process of LiFePO4 material mainly transforms
between LiFePO4 and FePO4 phases, and the volume change rate is small, which
makes the material extremely stable, so the safety and stability of lithium iron
phosphate materials and batteries are beyond doubt.
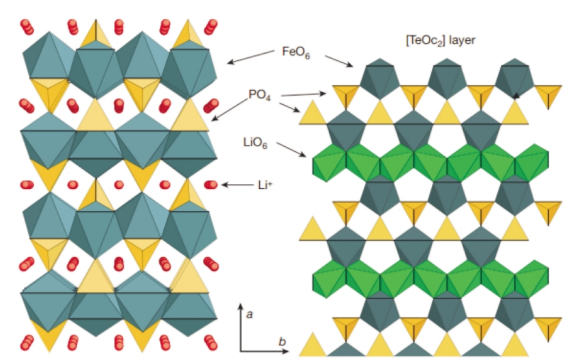
(Structural model diagram of lithium iron phosphate material)
Lithium iron phosphate batteries mainly have the following
characteristics:
(1) The cycle performance of lithium iron phosphate batteries is excellent,
and the cycle life of energy-type batteries can be as long as 3,000 to 4,000
times, and models with cycles that can even reach tens of thousands of times can
be manufactured according to customer needs;
(2) The lithium iron phosphate battery has excellent safety performance, it
can maintain a relatively stable structure even at high temperature, and even
when the battery is deformed and damaged, there will be no safety accidents such
as smoke and fire.
Lithium iron phosphate raw material resources are relatively abundant,
which greatly reduces the cost of materials and batteries. That is, in the
future, the price reduction ratio of lithium iron phosphate batteries will be
greater than that of ternary lithium batteries.
At the same time, since iron and phosphorus elements are environmentally
friendly, lithium iron phosphate materials and batteries do not pollute the
environment. This is also one of the reasons why lithium iron phosphate
batteries can completely replace lead-acid batteries.
The structural properties of LiFePO4 material determine that the material
has low ionic and electronic conductivity, and as the temperature decreases,
both the electron transfer resistance and the charge transfer resistance
increase rapidly, resulting in poor low temperature performance of the battery.
Of course, this problem has been overcome by SES Power, our 26650 lithium iron
phosphate battery with a capacity of up to 3400mah, it can discharge at a rate
of 3C at -40 degrees Celsius with an efficiency of 90%, and can be discharged at
-30 degrees Celsius at 3C Rate charging, and life is not affected, so it is
widely used in polar environments, such as electric snow sleds, snow electric
motorcycles, Antarctic and Arctic inspection instruments, outdoor monitoring
equipment in extremely cold environments, etc.
B: Ternary materials and batteries
Li(NixCoyMn1-x-y )O2 material has attracted great attention of researchers
since it was first reported, for the simple reason that it can reduce the amount
of Co used. This can reduce the cost pressure brought about by price
increases.
Li(NixCoyMn1-x-y )O2 has structural similarities with LiCoO2. Taking the
NCM111 ternary material as an example, Li+ is located at the 3a position in the
structure, Ni, Mn, and Co are randomly distributed at the 3b position, and
lattice oxygen occupies the 6c position. The transition metal layer structure is
composed of Ni, Mn, Co, and is surrounded by 6 lattice oxygens to form a MO6
(M=Ni, Co or Mn) octahedral structure, and lithium ions are inserted between the
MO6 layers. During the charging and discharging process, lithium ions are
deintercalated in the interlayer structure of MO6, and the electric pairs
participating in the electrochemical reaction are Ni2+/Ni3+, Ni3+/Ni4+ and
Co3+/Co4+, respectively, while Mn is electrochemically inert and does not
contribute to electrochemical reactions. capacity.
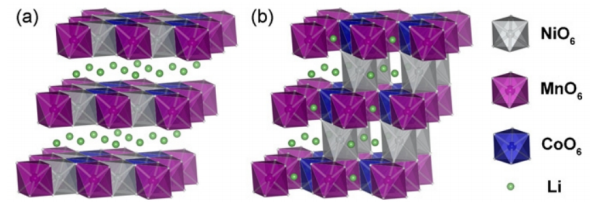
(Structure diagram of ternary material without Li/Ni shuffling (a) and with
Li/Ni shuffling (b))
According to the proportion of Ni content, ternary materials and batteries
can be divided into conventional type and high nickel type. With the increase of
Ni content, the deintercalable lithium increases, and the material capacity and
battery energy density increase. Therefore, high-nickel ternary materials and
batteries are the current research hotspot and full of challenges.
(1) Since the radius of Ni2+ is very close to the radius of Li+, with the
increase of Ni content, the probability of Li/Ni mixing in high-nickel ternary
materials increases sharply during high-temperature sintering preparation, and
the lithium deintercalation into the MO6 layer is more difficult. It hinders the
Li+ transport ability, resulting in lower specific capacity and lower cycle
performance, which is difficult to reverse.
(2) With the increase of Ni content, the proportion of Ni3+ in the material
also increases, and Ni3+ is very unstable, and it is very easy to react with
moisture and CO2 in the air to generate surface residual alkali when exposed to
air, resulting in the capacity of ternary materials. and cycle performance loss.
In addition, too much residual alkali on the surface will cause serious gas
production in the ternary battery, affecting its cycle performance and safety
performance.
(3) The high-valent Ni element also has high catalytic activity and
oxidizing property, which leads to the decomposition of the electrolyte and also
causes the battery to produce gas. In order to solve the above problems,
customization of precursors, individualization of sintering process, ion doping,
surface coating modification, wet processing and production environment control
have become common choices for ternary material manufacturers.
For the ternary battery, its performance characteristics mainly include
high material mass specific capacity, mass and volume specific energy, good rate
performance and low temperature performance, but due to the stable structure and
scarcity of nickel and cobalt resources, its cycle Better performance, general
safety performance, and higher cost.
C: Detailed comparison and analysis of two materials and batteries
c.1 Energy density
Compared with lithium iron phosphate materials, ternary materials have
higher discharge specific capacity and higher average voltage, so the mass
specific energy of ternary batteries is generally higher than that of lithium
iron phosphate. In addition, due to the low true density, small particle size
and carbon coating of the lithium iron phosphate material, the compacted density
of the pole piece is about 2.3-2.4 g/cm3, while the compacted density of the
ternary pole piece can reach 3.3 ~3.5 g/cm3, so the volume specific energy of
ternary materials and batteries is also much higher than that of lithium iron
phosphate.
c.2 Security
The main structure of the lithium iron phosphate material is PO4, and the
bond energy is much higher than the M-O bond energy of the ternary material MO6
octahedron. The thermal decomposition temperature of the fully charged lithium
iron phosphate material is about 700 °C, while the thermal decomposition
temperature of the corresponding ternary material is 200 to 300 °C, so the
lithium iron phosphate material is safer.
From a battery perspective, lithium iron phosphate batteries can pass all
safety tests, while ternary batteries cannot easily pass tests such as
acupuncture and overcharge, and need to be improved from structural parts and
battery design.
c.3 Power performance
The activation energy of Li+ of the lithium iron phosphate material is only
0.3-0.5 eV, resulting in a Li+ diffusion coefficient of the order of 10-15-10-12
cm2/s. The extremely low electronic conductivity and lithium-ion diffusion
coefficient lead to the poor power performance of LFPs. The Li+ diffusion
coefficient of the ternary material is about 10-12 ~ 10-10 cm2/s, and the
electronic conductivity is high, so the ternary battery has better power
performance.
c.4 Temperature suitability
Affected by the low electronic conductivity and ionic conductivity of the
lithium iron phosphate material, the low temperature performance of the lithium
iron phosphate battery is poor. Compared with normal temperature, the capacity
retention rate of lithium iron phosphate battery at -20 ℃ discharge is only
about 60%, while the ternary battery of the same system can reach more than
70%.
Of course, this problem has been solved by SES Power.
c.5 Cost and Environmental Factors
Ternary materials contain rare metals such as Ni and Co, and the cost is
higher than that of lithium iron phosphate. With the improvement of materials
and battery technology, the cost of ternary and lithium iron phosphate batteries
has dropped significantly. At present, the market price of ternary batteries is
higher than that of lithium iron phosphate batteries.
At the same time, compared with the environmentally friendly Fe and P
elements, the Ni and Co elements in the ternary materials and batteries are more
polluting to the environment. Combined with the above factors, the demand for
environmental control and waste recycling of ternary materials and batteries is
more urgent.




































