The concept of sodium battery was actually first proposed by the French
science fiction writer Verne in the famous science fiction novel "Twenty
Thousand Miles Under the Sea" in 1870. In the novel, the submarine Nautilus
takes the electrolyte sodium chloride in the sea water and makes a sodium
battery as an energy source to drive it forward.
Lithium batteries have long been widely used in production and life, such
as power batteries as the core component of new energy vehicles; but the
development of sodium batteries has not been very smooth. For a long time, only
some high-temperature sodium-ion batteries have been used in small-scale energy
storage power stations and low-speed in the automotive field, even in 2011,
research on room temperature sodium-ion batteries began to revive, and only in
recent years have companies attempted to commercialize products.
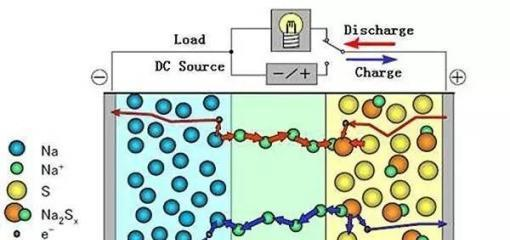
Sodium ion battery, short for sodium battery, as the name implies, is a
battery driven by sodium ion. A kind of power battery that realizes the movement
of electric charge through the "swimming" of sodium ions between the positive
and negative electrodes of the battery.
Why was the sodium ion battery once left out? This is actually very
directly related to its chemical properties. In fact, sodium batteries and
lithium batteries were born together because their working principles are
similar. The atomic weight of lithium is 6.94, which is the lightest among
metals; in addition, the specific capacity of lithium is also the highest among
metals, and its electrochemical equivalent is the smallest. This means that
lithium batteries can theoretically obtain the greatest energy density. In the
battery field, if safety and cost factors are not considered for the time being,
energy density is the most representative indicator of battery performance.
Therefore, lithium batteries were the first choice in the eyes of developers at
the time. The sodium ion battery was once forgotten by researchers in the
corner. It was not until after 2010 that the room temperature sodium ion battery
returned to the researchers' field of vision.
Looking at the periodic table, the metal element closest to lithium is
sodium. They are all located in the first column of the periodic table, with the
same number of electrons in the outermost layer and similar chemical properties,
so they can form the first oxidation state and act as a charge transporter to
drive the battery to charge and discharge. However, compared with lithium-ion
batteries, sodium-ion batteries have obvious shortcomings. The first is energy
density. The reason is that sodium ions are heavier than lithium ions, and their
electronegativity is not lower than that of lithium. Therefore, the same
electrode material usually has a lower voltage and a lower specific capacity
than the corresponding lithium ions, which leads to the energy density of the
battery. Lower. On the other hand, since the radius of sodium ions (0.102nm) is
more than 30% larger than the radius of lithium ions (0.076nm), sodium ions are
relatively stable in a rigid structure and it is difficult to reversibly
deintercalate. Even if deintercalation can occur, the kinetics of insertion and
deintercalation of sodium ions are very slow, which can easily cause
irreversible phase changes in the electrode material structure and reduce the
cycle performance of the battery.
As a result, when the energy density of advanced ternary lithium batteries
is above 200Wh/kg, sodium-ion batteries are only 100-150Wh/kg. Even if the
energy density of advanced sodium-ion batteries released by CATL can reach
160Wh/kg, it is comparable to lithium-ion batteries. The battery gap is also
obvious.
In this way, in the 1980s when science and technology were not yet
developed, lithium-ion batteries and sodium-ion batteries took a completely
different path: the former was quickly commercialized and became an
indispensable product in the consumer market, while the latter completely
entered the market. The state of stagnation.

Lithium-ion battery (LIB) has become the main energy storage solution in
modern social life. Among them, lithium iron phosphate battery is a perfect
replacement for lead-acid batteries, and it is the first choice for
grid-connected peak shaving, off-grid energy storage, photovoltaic energy
storage, UPS, data center and other industries.




































