Over time, the loss of oxygen in the lithium ion electrode will impair
battery performance
We know that a lithium-ion battery is essentially a chemical battery, so it
is necessary to carefully and extremely accurately measure changes in material
properties during research.
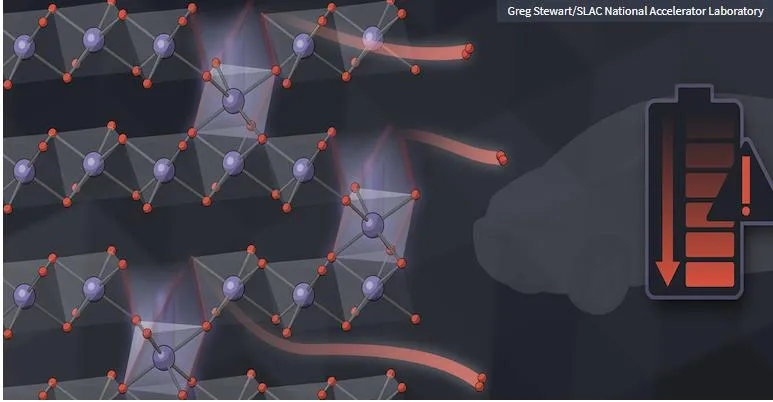
The picture shows: SLAC and Stanford University scientists have carried out
detailed, nanometer-level measurements on how the oxygen that constitutes the
electrodes of lithium-ion batteries can leak out. In the picture, the red
spheres are oxygen atoms that ooze out, and the purple spheres are metal
ions.
As we all know, when lithium ions flow in the process of charging and
discharging, the voltage of the battery will drop, and various components will
also be very slightly lost. Over time, the accumulation of these losses will
cause the battery's energy storage to easily decrease by 10-15%. During the
battery charge and discharge cycle, researchers at the SLAC National
Acceleration Laboratory (a national laboratory under the U.S. Department of
Energy, operated and managed by Stanford University) discovered that a very
small amount of oxygen leaked out. They made very detailed measurements of this
ultra-slow process.
Ph.D Peter Csernica of Stanford University said: "In the experiment in
collaboration with Associate Professor Will Chueh, we measured a very small
amount of oxygen outflow over hundreds of cycles, the rate is very slow, which
is why it is difficult to find." Researchers have found that sometimes, as
lithium moves back and forth, oxygen atoms are lost among the billions of
particles that make up each electrode. It is difficult to measure these oxygen
losses. Csernica said: "The total amount of oxygen loss reached 6% in more than
500 charge and discharge cycles. This is not a small number, but if you try to
measure the amount of oxygen loss in each charge and discharge cycle, it is
about 1‰. "
The traditional view is that the loss of oxygen only comes from the surface
of the nanoparticles. SLAC researchers are studying how oxygen loss changes the
chemical properties and structure of particles, rather than trying to directly
measure the rate of oxygen loss. The research team concluded: The initial loss
of oxygen starts from the surface, and then slowly flows out from the inside.
When the nanoparticles are condensed into a whole to form a larger cluster,
those near the center will lose less oxygen than the surface.
Chueh pointed out another very important issue: how the loss of oxygen
atoms affects other materials. Chueh said: "This is actually a big mystery.
Imagine that the atoms in a nanoparticle are like closely packed spheres. If you
keep taking out oxygen atoms, everything will collapse due to structural
changes." The research team said: “From the aspect of electrode structure, it is
impossible to directly image. We have carried out computer simulations and
calculations with other types of experimental observations and various oxygen
loss scenarios. The results show that holes do exist, but the material does not
the structure collapses and densification occur. This shows from another
perspective that the loss of oxygen will lead to a gradual decrease in battery
power." Chueh said: "When oxygen seeps out, the surrounding manganese atoms,
nickel atoms and cobalt atoms also move with it. All atoms deviate from the
ideal position. The chemical changes caused by the recombination of this metal
ion with the loss of oxygen will gradually reduce the voltage and efficiency of
the battery over time. We have known these surface phenomena for a long time,
but I don’t know the real root cause."

Lithium-ion battery (LIB) has become the main energy storage solution in
modern social life. Among them, lithium iron phosphate batteries are a perfect
substitute for lead-acid batteries and are the first choice for grid-connected
peak shaving, off-grid energy storage, photovoltaic energy storage, UPS, data
center and other industries.




































