Lithium-ion secondary batteries (LIBs) are the mainstream energy storage
devices in the field of new energy today. Lithium iron phosphate (LiFePO4) has
become one of the most widely used cathode materials for lithium-ion batteries
due to its high energy density, low cost, stable charging and discharging
platform, environmental friendliness, and high safety.
As a manufacturer with nearly 20 years of experience in lithium battery
customization services, SES Power has been paying attention to the development
of the lithium battery industry, especially the development of lithium battery
cathode materials. Because the cathode material is the core of almost all raw
materials of lithium batteries. When SES Power first made a lithium battery pack
in 2008, it used the 18650 model of lithium iron phosphate battery with a
capacity of only 1200mah. By 2021, the capacity of this model can reach 2000mah,
which is almost doubled. times. Our energy storage products, such as 12V100Ah,
12V200Ah, 24V100Ah, 24V200Ah, 36V100Ah, 48V50Ah, 48V100Ah, and the home energy
storage system HESS (3KW inverter output) using square aluminum lithium
batteries, were unimaginable ten years ago. A single cell reaches 100Ah or even
280Ah. Similarly, SES Power's custom lithium-ion batteries can now be used in
-60 degrees and -40 degrees Celsius. These products were almost unimaginable
before.
With the development of the new energy industry, customers have put forward
higher requirements for the electrochemical performance of batteries such as
cycle stability, high-rate performance, and energy density. How to improve its
output power, energy density and service life at low temperature is the main
challenge for lithium iron phosphate cathode materials. According to the
research of SES Power, element doping improves the electrochemical performance
of the material, the protection mechanism of different capping agents for
lithium iron phosphate, and the high-capacity lithium-replenishing material.
These three methods can effectively improve the electronic conductivity and
ionic conductivity of lithium iron phosphate cathode materials. Diffusion rate
to achieve higher energy density, longer cycle life and higher rate capability
of the material.
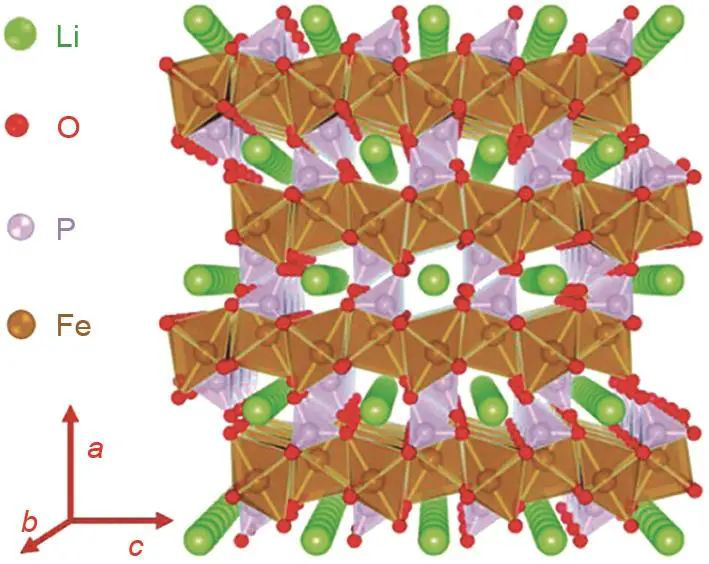
(Crystal structure of LiFePO4 viewed from the Li+ one-dimensional diffusion
channel)
A: Challenges faced by LiFePO4
LiFePO4 material has stable charging and discharging platform, high
specific capacity, good safety, less self-discharge, low cost and no pollution
to the environment. However, LiFePO4 is limited by its own crystal structure,
poor conductivity and slow ion migration rate, which greatly limit the
improvement of its electrochemical performance. In a sub-zero temperature
environment, the energy density of LiFePO4 batteries decreases, shortening the
battery life and increasing the operating cost of the battery system.
B: LiFePO4 improvement method
Due to the low ion diffusion rate and poor electrical conductivity of
LiFePO4, it has a great impact on the rate capability and low temperature
performance of LiFePO4. Therefore, how to improve the ion diffusion rate and
electrical conductivity of materials, so as to further effectively improve the
rate performance and low temperature performance, has always been the focus of
researchers.
b.1 Element doping modification
Element doping is considered to be an important method to improve the
internal electronic conductivity and ionic diffusivity of LiFePO4 materials.
This method can improve the charge-discharge performance of LiFePO4 material at
high current density.
The electrochemical data suggest that a moderate amount of Na doping can
improve the electrochemical performance. Li0.99Na0.01FePO4 exhibits excellent
rate performance and cycling stability, with an initial discharge specific
capacity of 80.9 mA h/g at a current density of 10 C and a capacity retention
rate of 86.7% after 500 cycles.
A small amount of Mn doping can improve the electrochemical performance of
the material, but too much doping will cause Li/Fe inversion defects and even
destroy the material structure. Although LiFePO4 doped with Mn element has
better electrical conductivity, its structural stability is relatively poor.
With the increase of Mn doping amount, in addition to the decrease in rate
performance, the dissolution of Mn2+ will change the structure of the cathode
material and reduce the discharge capacity.
Two-element doping is a good method, and Li site doping and Fe site doping
are usually the most studied.
Multi-ion co-doping mainly incorporates two or more metal elements into the
LiFePO4 structure, and integrates the advantages of each doped metal ion to
improve the electrochemical performance.
All in all, element doping can improve the internal conductivity of LiFePO4
material particles and accelerate the diffusion of lithium ions, and is still
the mainstream choice for optimizing material properties.
b.2 Material cladding
To prepare LiFePO4 with excellent electrochemical performance, only doping
is not enough. The conductivity of LiFePO4 is extremely poor. By coating the
surface of the material with a suitable conductive/ion-conducting material, the
electronic and ionic conduction between the particles of LiFePO4 can be
improved.
The types of coating agents mainly include carbon materials, metal or metal
oxide materials, and ion conductive materials. Among them, coating LiFePO4
material with conductive material is an important measure to improve its rate
and low temperature performance, and carbon material is the simplest and
cheapest kind of excellent material.
Graphene has the advantages of high electrical conductivity and porous
structure, and it is also a good direction. The improved LiFePO4 with graphene
has excellent cycling and rate performance. The discharge specific capacity was
160 mA h/g at 0.2 C rate, 107 mA h/g at 60 C high rate, and the capacity
retention rate over 2000 cycles was 95%.
Metal or metal oxide and carbon composite metal material coating is also
feasible, the battery conductivity is significantly improved, and the tap
density is also improved. However, the metal coating has an oxidation problem,
and the metals introduced are generally precious metals, which are not suitable
for mass production.
Ion conducting materials can also be used to improve LiFePO4 cathode
materials.
b.3 Adding Lithium Supplementary Materials
During the first charging process of LiFePO4 batteries, due to the
formation of a solid electrolyte interface (SEI) on the surface of the negative
electrode, about 5% to 20% of the lithium in the positive electrode material is
consumed, resulting in excessive irreversible capacity loss. We can add lithium
supplementary material to the lithium iron phosphate positive electrode
material. During the charging process of the battery, the lithium supplementary
material decomposes and releases excess lithium to compensate for the
irreversible lithium loss caused by the formation of the SEI film on the
negative electrode. Lithium supplementation materials usually have the
characteristics of strong lithium supplementation ability, easy synthesis,
strong stability and low cost. Common lithium iron phosphate cathode
supplementary materials include Li2O, LiF, Li3N and Li2S.
C: Summary and Outlook
After nearly 30 years of development, LiFePO4, as a commercialized cathode
material, still has many aspects worthy of further research. In the future, the
research on LiFePO4 cathode materials can focus on the following aspects.
(1) Industrialized production improvement. The cathode material of LiFePO4
has been industrially produced, but there are disadvantages such as high energy
consumption and poor rate performance of the produced material, and there is
still a lot of room for improvement.
(2) The combination of various improvement strategies can further improve
the rate performance of LiFePO4, and realize the ultra-fast charging
characteristics and excellent low-temperature long-cycle performance of the
material.
(3) Further in-depth theoretical research, to study the thermodynamic and
kinetic transformation process of LiFePO4 material in the process of charging
and discharging.
(4) Develop solid-state electrodes. Combining the LiFePO4 cathode material
with a solid electrolyte produces a new type of lithium battery that is safer
and can be used in flexible wearable electronic devices.
SES Power believes that LiFePO4, a cathode material for lithium ion
batteries, has broad application and development prospects in the commercial
field. It is believed that through the joint efforts of researchers, the further
performance improvement of LiFePO4 cathode material can maximize people's
production and life needs.



































