Analysis of the main reasons for the capacity degradation of lithium
batteries
It is an inevitable phenomenon that capacity attenuation and loss occur
during battery cycling. Therefore, in order to improve battery capacity and
performance, many scholars have studied the mechanism of lithium battery
capacity loss. At present, it is known that the main factors that cause the
capacity degradation of lithium-ion batteries include the formation of SEI
passivation film on the surface of the positive and negative electrodes, the
deposition of metal lithium, the dissolution of electrode active materials, the
occurrence of anode and cathode redox reactions or side reactions, structural
changes and phase changes, etc. At present, the capacity decay changes of
lithium-ion batteries and their causes are still under constant research.
(1) Overcharge
When a lithium-ion battery is charged and discharged for the first time, a
passivation film called SEI is formed. This reaction will cause the loss of
battery capacity and is an irreversible process. During the overcharging process
of lithium ion batteries, metal lithium deposition occurs on the surface of the
negative electrode, which is likely to occur when the positive electrode active
material is excessive relative to the negative electrode active material. At the
same time, under high-rate conditions, metal lithium deposition may also
occur.
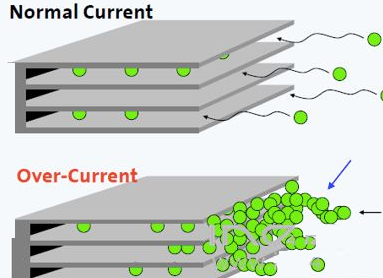
Generally speaking, metallic lithium will cause: first, the amount of
recyclable lithium in the battery is reduced; second, the side reaction of
metallic lithium with electrolyte or solvent, forming other by-products; third,
metallic lithium is mainly deposited on the negative electrode and separator In
between, the pores of the diaphragm are blocked, resulting in an increase in the
internal resistance of the battery.
The positive electrode overcharge mainly occurs when the proportion of the
positive electrode material is too low. This situation will cause the capacity
imbalance between the electrodes, resulting in irreversible loss of the lithium
battery capacity, and the coexistence of oxygen and combustible gas from the
decomposition of the positive electrode material and the electrolyte and
continuous accumulation may bring safety hazards to the use of lithium
batteries.
(2) Decomposition of electrolyte
The electrolyte includes electrolytes, solvents and additives, and its
properties will affect the battery's service life, specific capacity, rate
charge and discharge performance, and safety performance. The decomposition of
electrolyte and solvent in the electrolyte will cause the loss of battery
capacity. During the first charge and discharge, the formation of SEI film on
the surface of the negative electrode by solvents and other substances will
cause irreversible capacity loss, but this is inevitable. In general, the
occurrence of side reactions between the electrolyte and the positive and
negative electrodes of the battery, as well as the by-products produced, are the
main factors that cause the degradation of the battery capacity.
(3) Self-discharge
Lithium-ion batteries generally suffer from capacity loss. This process is
called self-discharge and can be divided into reversible capacity loss and
irreversible capacity loss.
The solvent oxidation rate has a direct effect on the self-discharge rate.
In addition, the diaphragm leakage will also cause a loss of capacity, but this
possibility is low. If the self-discharge phenomenon exists for a long time, it
will lead to the deposition of metal lithium, and further cause the attenuation
change of the positive and negative electrode capacity.
(4) Electrode instability
During the charging process, the active material of the positive electrode
of the battery is unstable, which will cause it to react with the electrolyte
and affect the battery capacity. Among them, the structural defects of the
positive electrode material and the excessively high charging potential are the
main factors that affect the battery capacity.
LiFePO4 has a wide range of sources, is cheap, and has good stability and
safety performance. It can reach a theoretical specific capacity of 170mAh/g,
and its specific power and specific energy are similar to LiCoO2, and can
achieve good compatibility with electrolyte solutions. Therefore, It is widely
used in the positive electrode of lithium battery.
Using this material, the main factors affecting the battery capacity
include the following two points: one is due to side reactions between the
positive and negative electrodes, resulting in the reduction of recyclable
lithium, which severely disrupts the balance between the positive and negative
electrodes; Factors such as structural deterioration, electrode delamination,
material dissolution, particle segregation, etc., lead to loss of active
materials, thereby affecting battery capacity.

Lithium-ion battery (LIB) has become the main energy storage solution in
modern social life. Among them, lithium iron phosphate batteries are a perfect
substitute for lead-acid batteries, and are the first choice for grid-connected
peak shaving, off-grid energy storage, photovoltaic energy storage, UPS, data
center and other industries.




































